Background: The Bruton tyrosine kinase (BTK) inhibitor zanubrutinib has demonstrated greater selectivity for BTK versus other TEC- and EGFRfamily kinases in biochemical assays, and favorable pharmacokinetic/pharmacodynamic properties in preclinical studies. In a phase 1 clinical trial, zanubrutinib showed complete and sustained 24-hour BTK occupancy in both peripheral blood mononuclear cells and lymph node biopsies from patients treated with 160 mg twice daily (Tam et al. Blood 2016;128:642). Zanubrutinib was also associated with high and durable responses in patients with WaldenstrC6m macroglobulinemia (WM) (Tam, ASCO 2020, abstract 8007; Dimopoulos, EHA 2019, abstract PF487; Trotman, EHA 2019, abstract PF481). Here we present initial safety and efficacy data from a phase 2 trial of zanubrutinib in patients with relapsed or refractory (R/R) WM.
Methods: BGB-3111-210 (ClinicalTrials.gov: NCT03332173) is a pivotal, single-arm, open-label, multicenter, phase 2 study conducted in China. Patients with R/R WM aged b %18 years and with b %1 prior line of standard chemotherapy-containing regimen (with completion of b %2 continuous treatment cycles) receive zanubrutinib 160 mg twice daily until disease progression or unacceptable toxicity. The primary objective is to evaluate the efficacy of zanubrutinib as measured by major response rate (MRR), defined as the proportion of patients achieving complete response (CR), very good partial response (VGPR), or partial response (PR), as assessed by an independent review committee (IRC) according to an adaptation of the 6th International Workshop on WM response criteria. Secondary endpoints include progression-free survival (PFS), overall response rate (ORR), duration of major response, and safety. The safety analysis set includes all patients who received b %1 dose of zanubrutinib. All efficacy endpoints are analyzed in patients with pathologically confirmed WM and with baseline immunoglobulin M (or M-protein) b %5 g/L. Adverse event (AE) severity is graded according to NCI CTCAE v4.03.
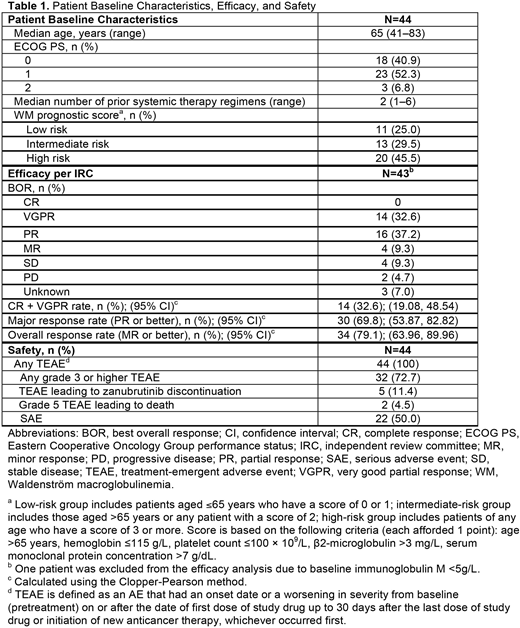
Results: As of August 31, 2019, a total of 44 patients with R/R WM were enrolled and treated, with a median follow-up of 18.58 months. Baseline characteristics are summarized in Table 1. For the 43 patients evaluable for efficacy (1 patient was excluded from the efficacy analysis due to baseline immunoglobulin M <5g/L), MRR per IRC was 69.8% (n=30, Table 1), with b % VGPR achieved in 32.6% of patients (n=14). As of data cutoff date, median PFS and median duration of major response were not reached. The most frequently reported (b %20%) treatment-emergent AEs (TEAEs) included neutrophil count decreased (56.8%), platelet count decreased (29.5%), white blood cell count decreased and upper respiratory tract infection (27.3% each), diarrhea (25.0%), weight increased and arthralgia (20.5% each). Grade 3 or higher AEs reported in b %5% of patients included neutrophil count decreased (31.8%), platelet count decreased (20.5%), lung infection (13.6%), white blood cell count decreased (11.4%), pneumonia (9.1%), anemia and upper respiratory tract infection (6.8% each). Most bleeding events were grade 1 or 2 originating in skin or mucous membranes. Major hemorrhage (serious or grade b %3 bleeding, or central nervous system bleeding of any grade) was reported in 2 patients (4.5%). No cases of atrial fibrillation/flutter or tumor lysis syndrome were reported. Two patients died within 30 days of last study treatment: one due to acute hepatitis B and multiple organ dysfunction syndrome, both of which were related to study drug per investigator's judgment, the other due to unknown reason, which was unlikely to be related to study drug as assessed by the investigator. TEAEs leading to discontinuation of zanubrutinib (n=1 each) included lung infection, laryngeal cancer, WM (investigator reported WM as AE due to unexpected rapid progression and suspected it to be a transformation), intracranial mass, acute hepatitis B (grade 5), and multiple organ dysfunction syndrome (grade 5).
Conclusions: Zanubrutinib was shown to be highly active in patients with R/R WM, as demonstrated by a high MRR, b %VGPR rate. Zanubrutinib was generally well tolerated, consistent with previous reports of zanubrutinib treatment in patients with various B-cell malignancies.
Zhou:Henan Cancer Hospital: Current Employment. Guo:BeiGene Co., Ltd.: Current Employment, Current equity holder in private company. Du:BeiGene Co., Ltd.: Current Employment, Current equity holder in private company. Huang:BeiGene: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Novotny:BeiGene USA, Inc.: Current Employment, Current equity holder in private company. Zhong:BeiGene Co., Ltd.: Current Employment, Current equity holder in private company.
Zanubrutinib is a small molecule inhibitor of Bruton's tyrosine kinase (BTK) that is being evaluated as a monotherapy and in combination with other therapies to treat various B-cell malignancies. Zanubrutinib is approved in the United States to treat adult patients with MCL who have received at least one prior therapy and in China for the treatment of MCL in adult patients who have received at least one prior therapy and CLL or SLL in adult patients who have received at least one prior therapy. Zanubrutinib is not approved for use outside of the United States and China.
Author notes
Asterisk with author names denotes non-ASH members.